Abstract
Background: Inflammatory myofibroblastic tumor (IMT) is a rare soft tissue sarcoma in childhood, nearly 50% of children have ALK gene rearrangement. The standard treatment for this disease is surgical resection. Some children with clinical manifestations of diffuse invasive lesions cannot undergo surgery, these children can be treated with ALK inhibitors, combination chemotherapy or other regimens. However, there is no effective treatment for children with ALK-negative IMT who are not sensitive to chemotherapy. Here, we report a child with ALK-negative IMT who had multiple organ involvement and poor respond to chemotherapy treated by CAR-T, and achieved certain efficacy with 2 year survival now.
Objective: To observe the effect of CAR-T cell therapy on refractory inflammatory myofibroblastic tumor in children.
Patient and Methods: The patient was a 9-year-old boy who was diagnosed with inflammatory myofibroblastic tumor in 2019. The lesion extensively involved multiple organs such as intestinal, peritoneum, lymph nodes, epididymis, etc., which could not be surgical resection. Combined chemotherapy regimens were ineffective, and ALK gene rearrangement was negative, so ALK targeted therapy could not be used. Therefore, the patient was diagnosed as refractory case. The pathological sections of the children were screened by immune-histochemical staining, and GD2 and PMSA were selected as the therapeutic targets according to the staining intensity. Then the GD2 and PMSA CART cells were infused, and the clinical symptoms and the changes of tumor foci were observed during several time points after infusion.
Results: CART cells were transfused for 3 times in total. In February and May 2020, GD2 CART and PMSA CART were transfused respectively, and then GD2 CART was transfused again in May 2022. No chemotherapy was given after cell transfusions.
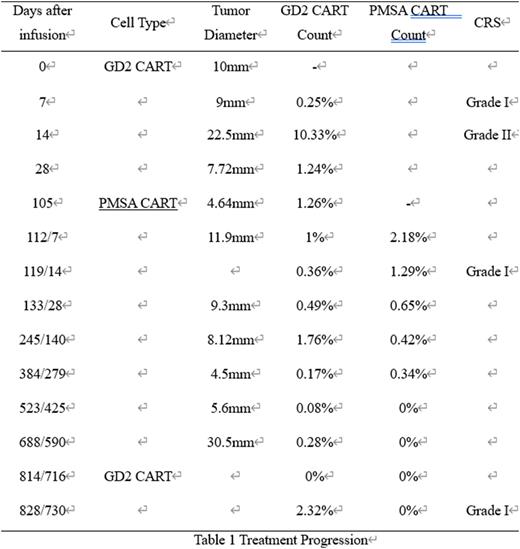
Treatment response: The clinical symptoms of the child patient including fever, abdominal distension were completely relieved in 30 days after the first GD2 CAR-T infusion. The tumor at the fixed position increased transiently after treatment and then gradually decreased. After infusion of 688 days, the tumor increased again, and a new tumor occurred accompanied by persistent high fever. It was considered that the primary disease was recurrent. The GD2 CART was reinfused after chemotherapy to control the symptoms. The child's temperature was controlled 30 days after re-treatment, and the new tumor decreased significantly. At present, the child is under continuous observation and follow-up (Table 1).
Blood CART monitoring: The peak of GD2 CART was detected on the 14th day after the first infusion and then gradually decreased. It could be detected in peripheral blood during the whole treatment process. The percentage of PMSA CART in blood reached the peak value on the 7th day after infusion, then gradually decreased and disappeared on the 425th day after infusion (Table 1).
Adverse effects: The overall adverse effects were controllable. After the first infusion of CART cells, grade II cytokine release syndrome (CRS) happened, manifested as fever and decreased oxygen saturation, which was improved after treatment with tocilizumab. After the second and third infusion,only Grade I CRS occurred, and it was relieved spontaneously. No other complications occurred such as tumor lysis syndrome, central nervous system toxicity, organ function damage and so on (Table 1).
Conclusion: the CART therapy of GD2 and PMSA is an effective treatment for ALK-negative diffuse inflammatory myofibroblastic tumor when the conventional chemotherapy has been failed. The targeted immunotherapy can control the tumor progress, prolong the survival time and improve the children's quality of life.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal